

By following a few simple rules and keeping in mind the exceptions, one can understand and communicate the names of any number of different ionic compounds. for sodium chloride NaCl you have a three dimensional array of positive sodium ions Na+ and negative chloride ions Cl. It is extremely important for chemistry enthusiasts to understand the naming convention of ionic compounds. These Ionic Compounds examples demonstrate the importance of understanding the different types of ionic compounds and their naming conventions in chemistry. Iron(III) refers to the cation with a +3 charge.Bicarbonate (HCO3) is the polyatomic anion.Ammonium (NH4) is the polyatomic cation.Copper(II) indicates the cation with a +2 charge.Iron(II) refers to the cation with a +2 charge.Hydroxide (OH) is the polyatomic anion. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal.
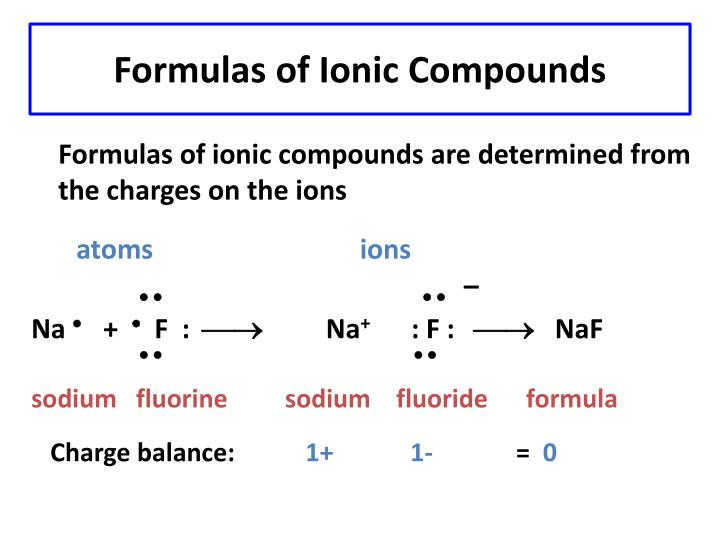

Polyatomic ions such as sulfate (SO42-) or hydroxide (OH-) have specific names to remember. Ionic compounds are neutral compounds made up of positively charged ions called cations and negatively charged ions called anions.A proper ionic formula has a cation and an anion in it an ionic compound is never formed. Some transition metals may have multiple charges, using Roman numerals to indicate the charge. Chemical formulas for ionic compounds are called ionic formulas.While the naming conventions for ionic compounds follow the rules outlined above, there are exceptions and additional considerations that must be taken into account: Common Exceptions and Additional Considerations 23) What is the formula of the compound formed. In cases where the cation has multiple oxidation states, Roman numerals are used to indicate the charge.įeCl2 is named iron(II) chloride, as Fe2+ is the cation and Cl- is the anion. 22) Of the choices below, which one is not an ionic compound A) PC15. NaCl is named sodium chloride, as sodium (Na+) is the cation and chlorine (Cl-) is the anion. If it can produce multiple charges, it is important to specify the charge of the cation. To name an ionic compound, the name of the cation and the name of the anion are combined.


 0 kommentar(er)
0 kommentar(er)
